Sketching a new product idea is one thing. Making it commercially viable is another. Here’s how Dr. Cosmo Haralambidis went from concept to market with his award- winning Synapse Dental Pain Eraser.
ON THE SURFACE, the idea seems simple: Create the dental equivalent of a TENS pain relief unit. What’s actually re-quired for it to work is inventing a true Class II neuromodulation device, not a simpler Class I muscle stimulator. There will be manufacturing and development challenges because the finished product has to be precise and small enough to work in the oral environment. And different versions should be priced affordably for both practitioners to use in-office as well as for patients to take home. Luckily, the idea popped into exactly the right person’s head.
Dr. Cosmo Haralambidis is a prominent Rhode Island ortho-dontist with a consulting background for innovative companies including 3M and EnvisonTEC. He has successfully brought his own products to market previously as well, such as, in 2009, Or-thoTracker, an early cloud-based interdisciplinary communica-tion tool. He even has materials-science chops and a cadre of big-name friends to brainstorm with—including, to name just one, trailblazing dean Dr. Scott De Rossi, No. 9 on this magazine’s 2023 list of the 32 Most Influential People in Dentistry. Here’s how Dr. Haralambidis made it work despite the pandemic, global supply chain disruptions and a lack of willing manufacturing partners.
Starting with a Clean Sheet of Paper
As an orthodontist, Dr. Haralambidis saw the need—and po-tential—for a product like his Dental Pain Eraser. “An effective, nonpharmacologic means of safe and portable pain control can revolutionize our field [and] change patient perceptions forever.” But, he points out, “anyone who has developed a medical product quickly realizes that it’s probably the hardest category to launch for new inventions, especially if it involves cell biology.” Even so, he wasn’t fully prepared for the challenges that lay ahead. One example: The Food & Drug Administration’s clearance process for a Class II medical device is multi factorial, in this case requir-ing several clinical validation studies with regard to efficacy and safety, because the Dental Pain Eraser has broad applica-tions for children and adults, both in the office and at home.
Refining the Concept
Dr. Haralambidis assembled teams to create prototypes for testing that were clinically useful and manufactur-able. “Baseline science knowledge required thousands of hours of literature review of the medical and dental journals, interviewing and surveying neurologists and surgeons who were experts in the field, and assessing the feasibility of clinical application,” he says.
Treating actual patients helped accelerate things somewhat. “Being a clinician offers the clinical eye of what will work in a specific setting when it comes to size, weight and cosmetic appeal to clinicians and patients,” he says. “The constant interaction with my independent patients and clinicians drove design adaptations with critique to design, shape and function issues. Initial clinical studies were performed in-office with the same rigor as compa-nies like 3M and university resident studies.”
Shopping It Around . . . and Around
Once the general shape, electronic system and de-livery probe mechanism were in place, the next log-ical step was partnering with one of the companies Dr. Haralambidis worked with in the past. Wouldn’t they be eager to grab the ball and run with it, es-pecially since the hardest work was already done? Not necessarily. “The industry has changed in that many large companies do not partner and create as one would visualize 50 to 100 years ago,” he says. Clearly, after coming this far, he wasn’t going to abandon the project so close to realization.

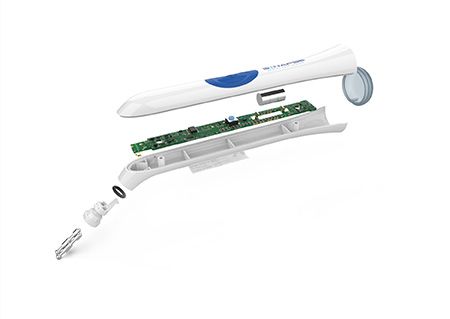
Deceptively simple: Like many good industrial designs—think of the iPhone and various smartwatches—a sleek and uncluttered exterior with intuitive controls shrouds the complex electronics within.
“The constant interaction
with my independent patients and clinicians drove design adaptations with critique to design, shape and function issues.”
—DR. COSMO HARALAMBIDIS
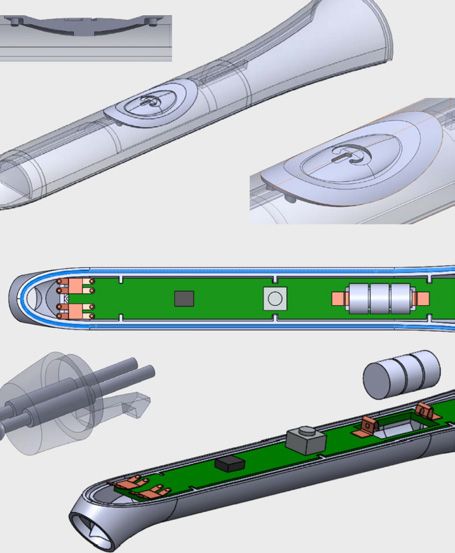
 Bringing It Home
Bringing It Home
Next, he assembled his own team and created a company, Synapse Dental. One key hire was George Aliphtiras, former head of a firm specializing in the commercialization of medical technol-ogy. “George [helped with] product market development for the first year and a half to assess strategy, and independently selected and hired [product development consultancy] Radius Innovation to bring my product design to the level of manufacture and FDA clearance,” Dr. Haralambidis says. In addition, Dr. Constantine Anagnostopoulos, a veteran electrical engineer from Kodak with nearly 80 patents to his name, served as an advisor. (Aliphtiras would eventually become Synapse Dental’s executive director and Dr. Anagnostopoulos its chief technology officer.)
 Off to Market
Off to Market
Without a megadollar launch budget, a lot of the marketing efforts had to be baked into the product itself. “It was designed to be self-evident in purpose both by its name, elegant pen-shape design and simplicity of function,” Dr. Haralambidis says. Building product awareness during the pandemic was a big lift, but grassroots user support and peer-to-peer influence helped. “Word spreads fast” when an innovative product comes along and fills a need, he adds.
Price, of course, was part of the equation too. At $299 for the clini-cal model, it’s accessible for practice owners, while the at-home device is only $85—a bargain considering a big bottle of generic ibuprofen costs $30. Making the at-home device even more appealingly marketable: It can be imprinted with custom office logos just like the toothbrushes you probably give away, and a tiered subscription plan offers significantly discounted pricing versus retail for added profit.
What’s Next
Since its launch, the Dental Pain Eraser has been highly rated by Clinicians Report and vetted by independent research. Peer- reviewed results of studies published in the Journal of Clinical Orthodontics showed that 44 of 45 subjects got relief, with 84 percent feeling pain relief within two minutes that lasted at least 48 hours. In addition, the American Association of Orthodontists honored Dr. Haralambidis with its 2023 Ortho Innovator Award, while the consulting firm Cellerant bestowed its Best of Class Hy-giene Award, which is voted on by a panel of prominent hygienists.
Mission accomplished? Not for Dr. Haralambidis. “We have new developments in process.” His secret to success also sounds like good advice for fellow doctors with big ideas. “First, be the expert in your field. Ensure you have the necessary educational skill set, mentorship and experience.” It also helps to have a thick skin. “Seek constant advice and guidance from colleagues, field experts and family. They are the best source to answer the honest ques-tion of whether a product is possible to bring to market.”
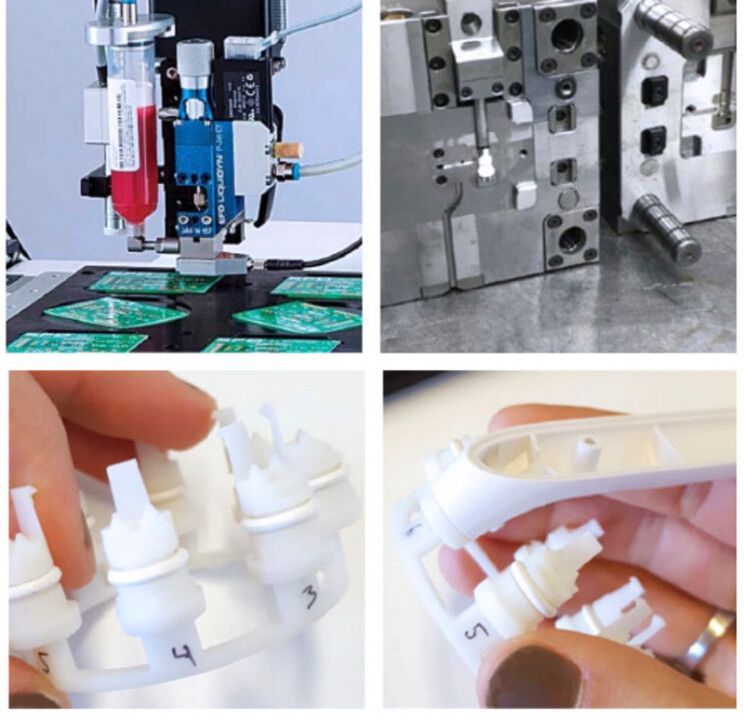
Painstaking process: Refining the printed circuit boards, creating molds and testing different replacement tip and adapter designs.




